american academy of pediatrics covid vaccine under 12
On June 17 the Food and Drug Administration approved the Pfizer and Moderna vaccine for children ages 6 months to 5 years. FDA authorization of the Pfizer-BioNTech COVID-19 Vaccine allows children who will turn from age 11 years to 12 years.

American Academy Of Pediatrics Ameracadpeds Twitter
AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical.

. The American Academy of Pediatrics is encouraged that we may be one step closer to a COVID-19 vaccine being available for children under age 5 pending a rigorous review of Pfizer. COVID Vaccines Authorized for Children Ages 6 Months Up. Have been fully vaccinated.
Lee Savio Beers immediate Past President of the American Academy of Pediatrics joins guest host Maggie Rodriguez on the nationally syndicated health and wellness. The AMA has announced an update to the CPT code set for new product and administration codes assigned to the Pfizer-BioNTech COVID-19 vaccine for children 6 months. The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021.
The American Academy of Pediatrics AAP recommends the COVID-19 vaccine for children in this. COVID Cases in Children Data Report. COVID-19 vaccines are finally authorized for babies age 6 months through 4 years old.
The label also states the vaccine must be discarded six hours after dilution but officials said the vaccine may be discarded 12 hours after dilution. How do we know COVID-19 vaccines are safe for kids. The Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and.
The American Academy of Pediatrics AAP is urging the US. Children who will turn from age 11 years to 12 years. American Academy of Pediatrics urges FDA to approve COVID vaccines for children under 12.
Both products come in. There are according to the American Academy of Pediatrics still five states where less than 20 of eligible 5- to 11-year-olds have received an initial vaccination. Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up.
A second COVID-19 vaccine for school-age children and adolescents moved closer to approval Thursday. Reassure parents and caregivers about the COVID-19 vaccine for children teens and young adults using resources from the AAP COVID-19 Vaccine Campaign Toolkit Share. Over half of all kids 12 to 17 years old in the US.
Children and COVID Vaccine Data Report. AAP calls for completion of clinical trials of the vaccine in younger ages to identify correct dose. Children under 5 may now get the COVID-19 vaccine.
Continues inoculating adults and adolescents questions remain. Training and Education for COVID-19 Vaccination. COVID-19 Vaccine Product Information.
NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID. Updated weekly this data report tracks the progress in vaccinating US children under 18 years of age. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12.
The Illinois Chapter of the American Academy of Pediatrics ICAAP and other partners have put together helpful handouts for. The Centers for Disease Control and Preventions CDCs Advisory. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full.
Recent research suggests that the Pfizer-BioNTech COVID-19 vaccine authorized for emergency use in children ages 5 to 11 isnt nearly as effective as it is for adolescents or. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. Neither the Johnson Johnson or Moderna vaccine have been.
Find a suite of information and materials needed for each specific COVID-19. Immediately following the American Academy. Thats over 13 million kids who have had both of their.
The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
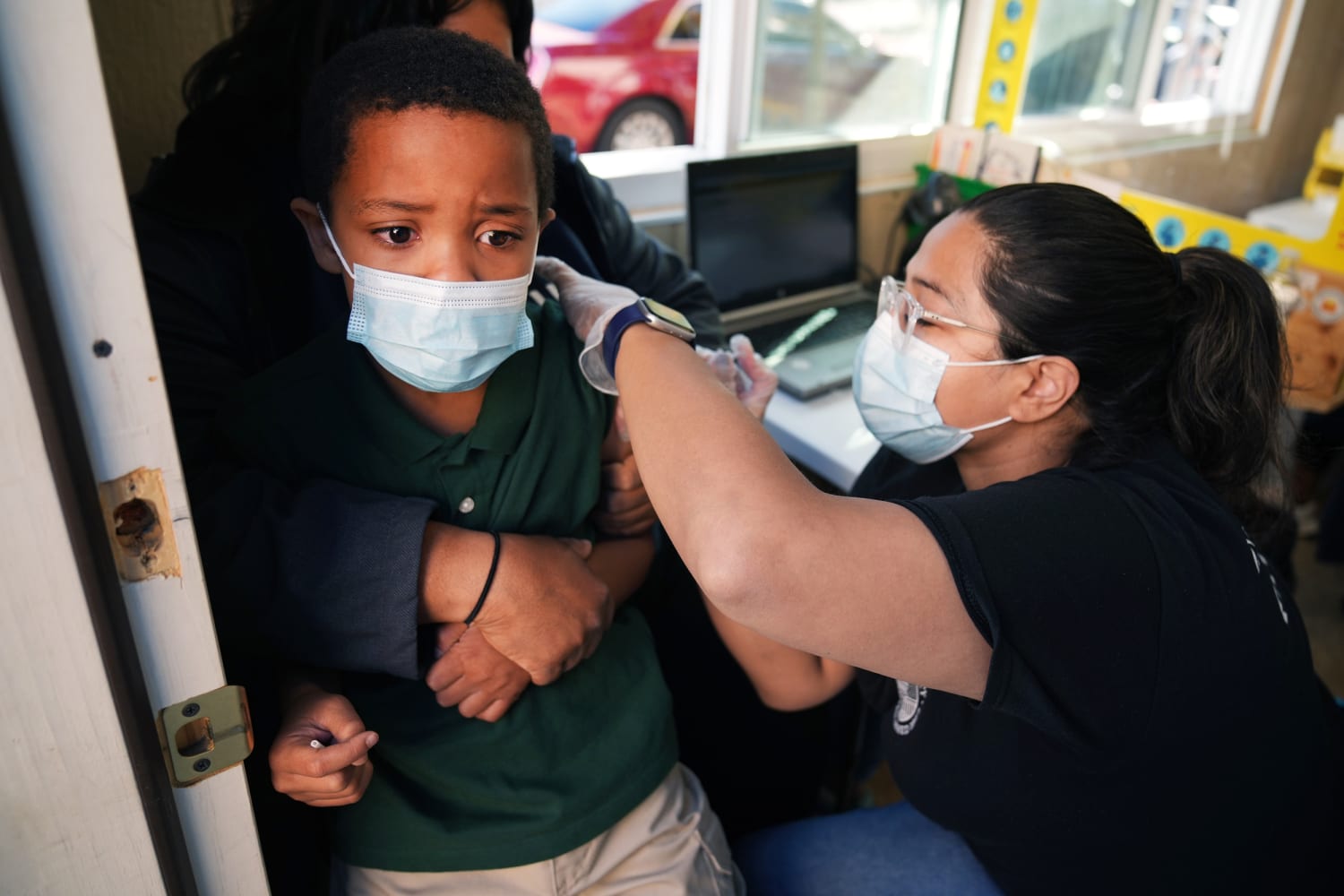
What Pediatricians Want Parents To Know About The Covid Vaccine For Kids

Kids Covid Vaccine Twitter Chat Twitter

Q A Aap Endorses Giving Routine Immunizations Covid 19 Vaccines At Same Time
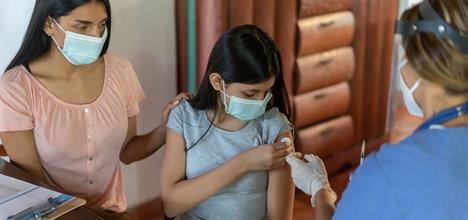
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
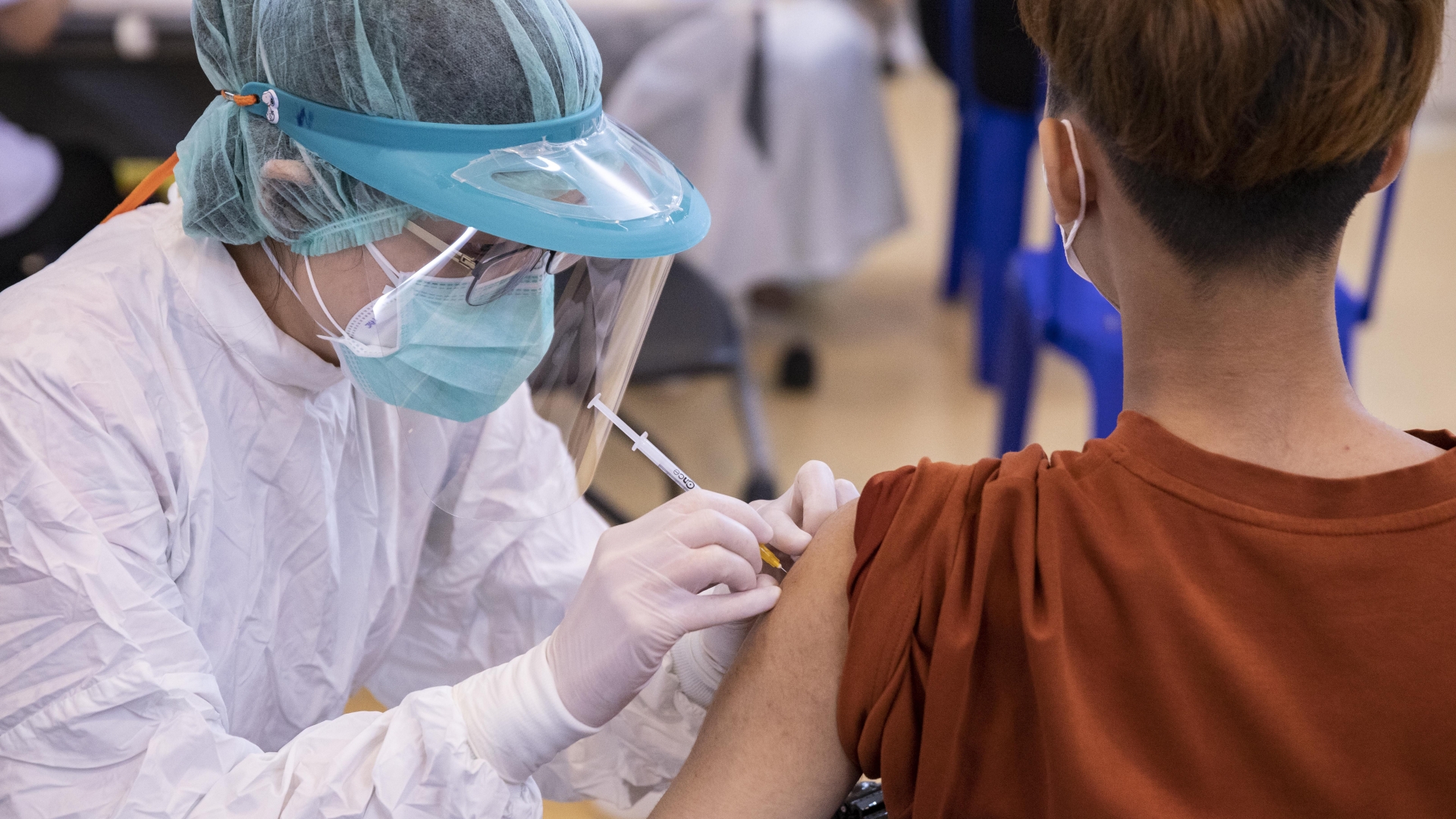
Pfizer Biontech Ask Fda To Authorize Coronavirus Vaccine For Children 5 To 11 The Washington Post
/Littlegirlgettingvaccineshot-e03a1d56400b45b1845ad8a4d22f5802.jpg)
The Covid 19 Vaccine Does Not Affect Kids Fertility

White House Calls On Pediatricians To Help With Rollout Of Kids Covid Vaccine
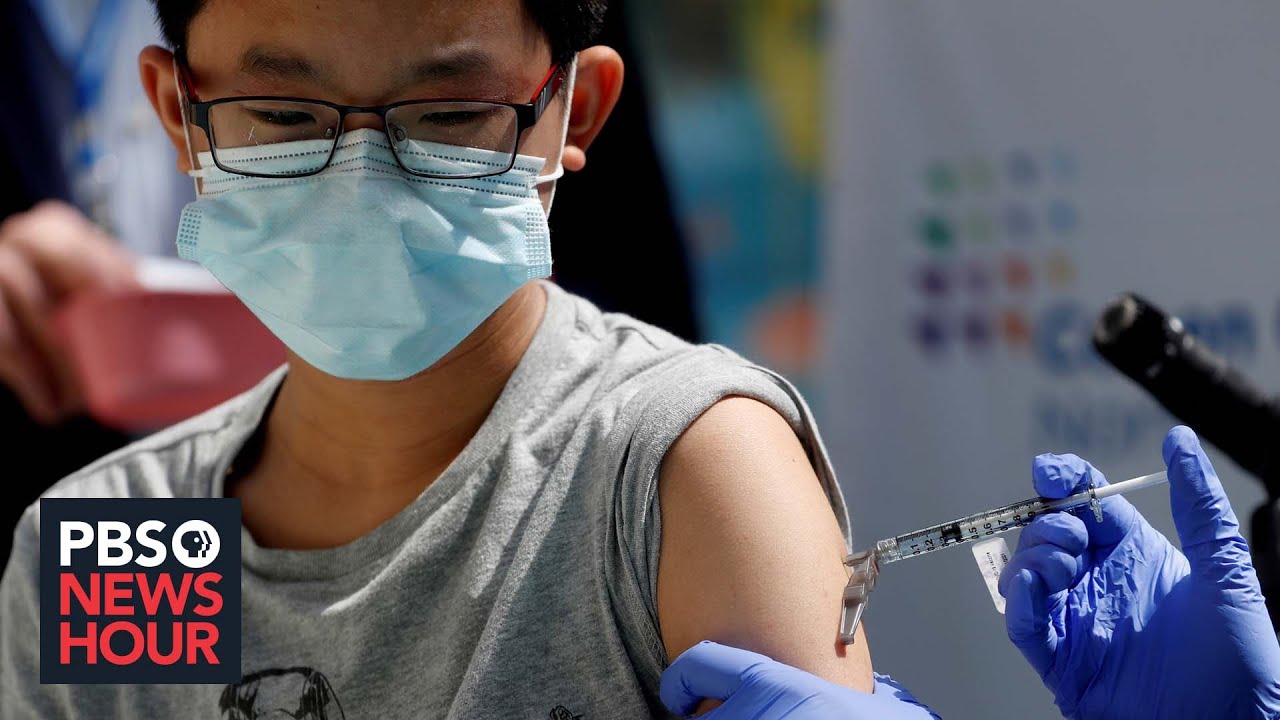
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
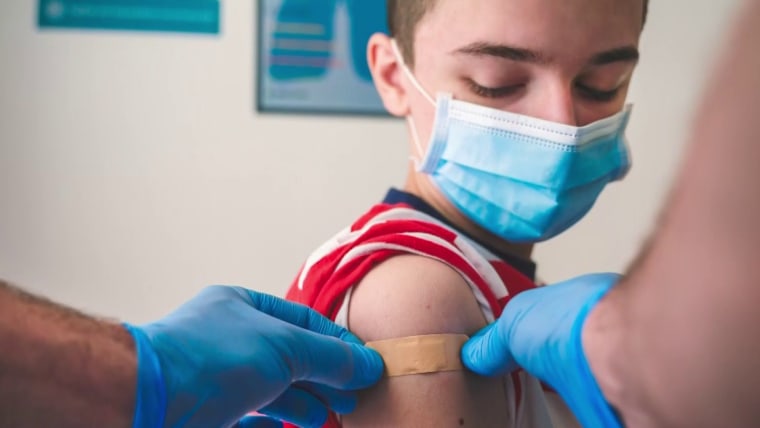
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr
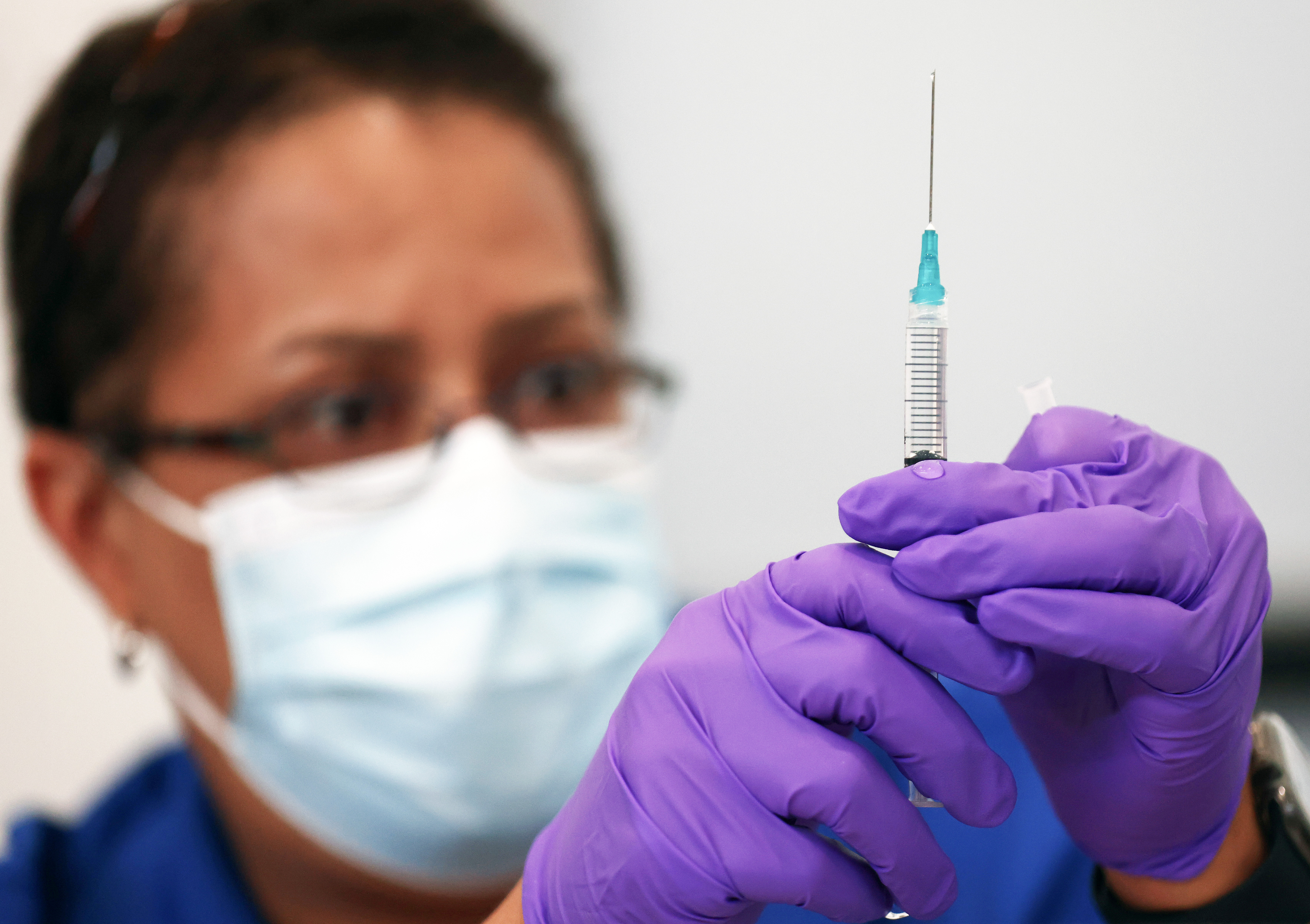
Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post
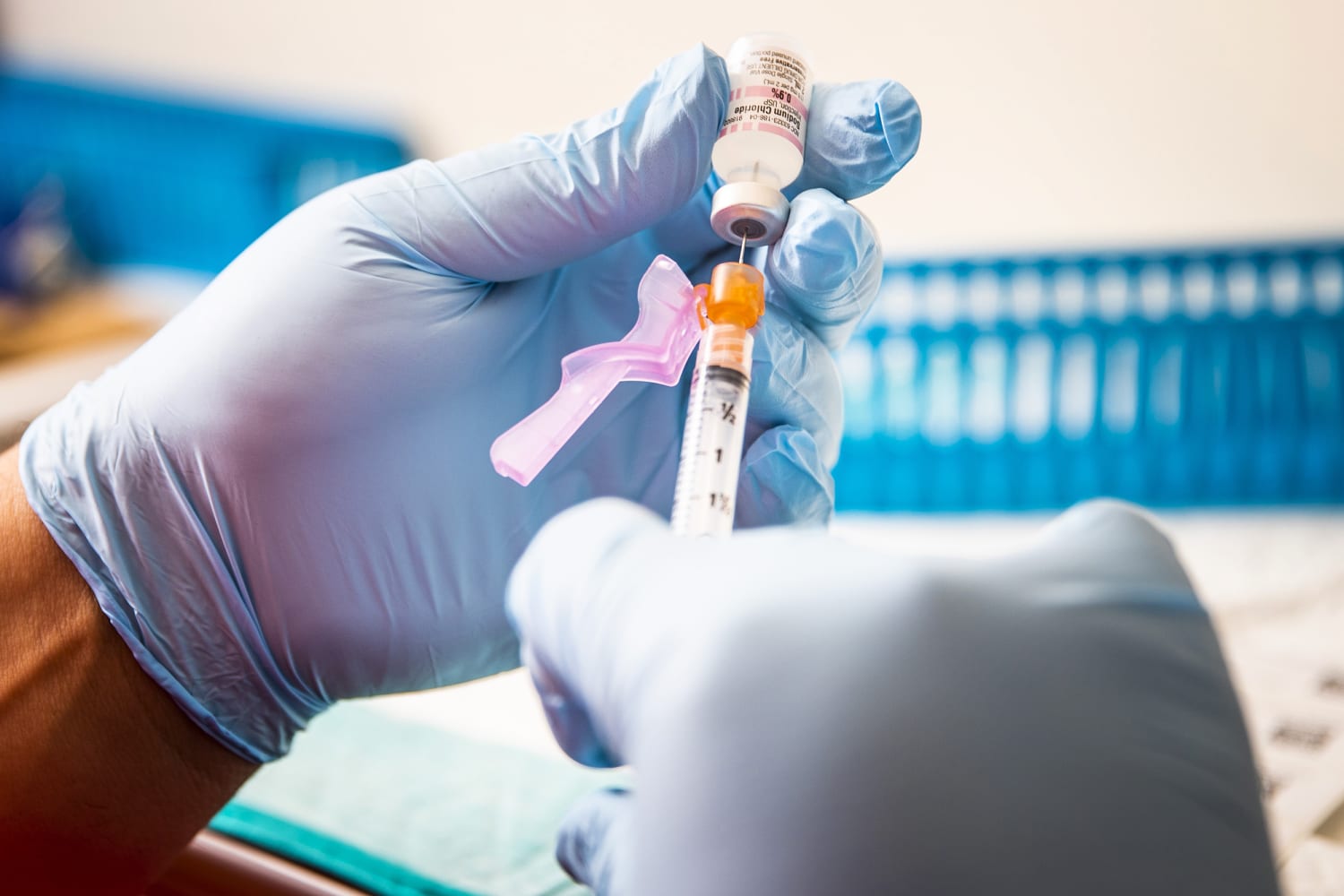
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
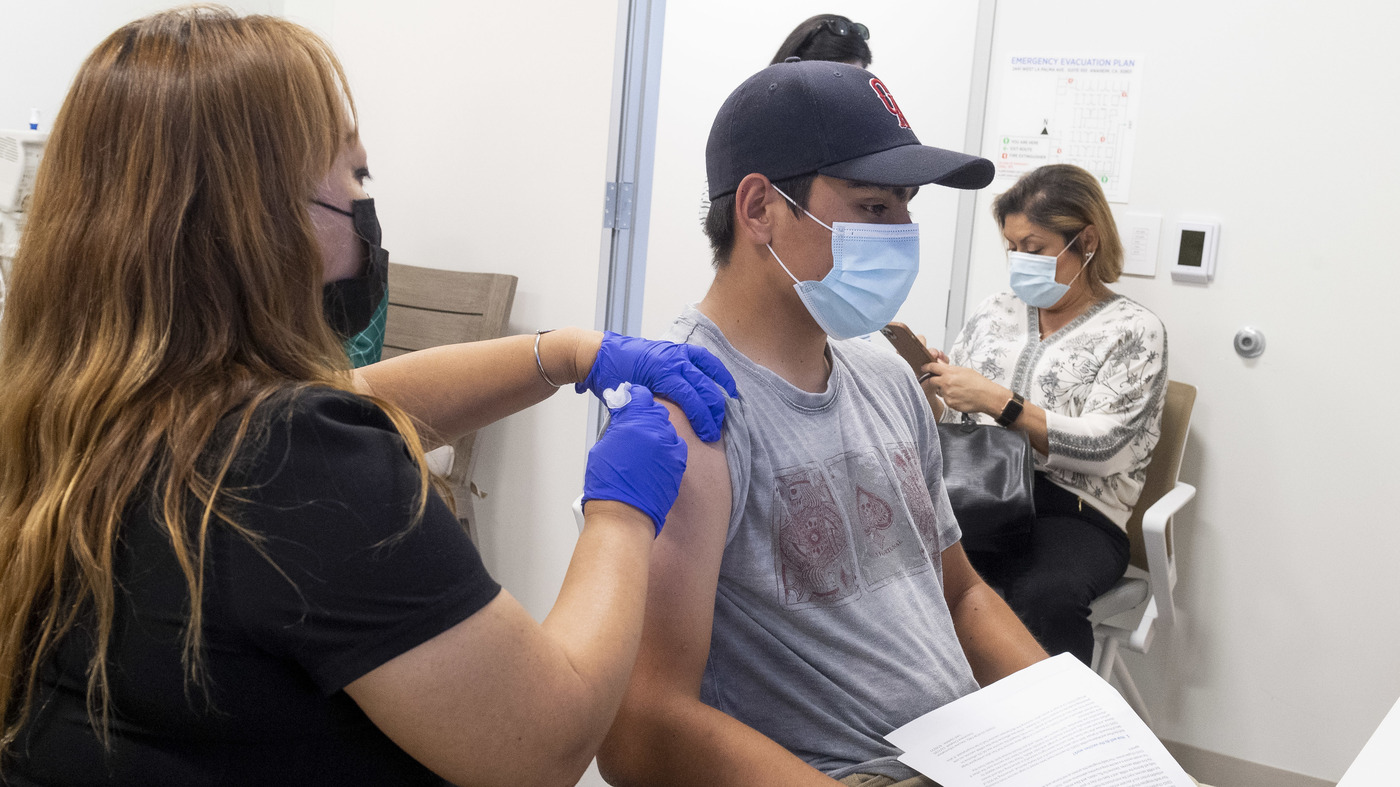
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine
Kids Covid Vaccine Side Effects What To Know

American Academy Of Pediatrics Los Ninos Y Los Adolescentes De 12 Anos En Adelante Ahora Pueden Recibir La Vacuna Contra El Covid 19 Haga Vacunar A Sus Ninos Para Que Puedan Volver

